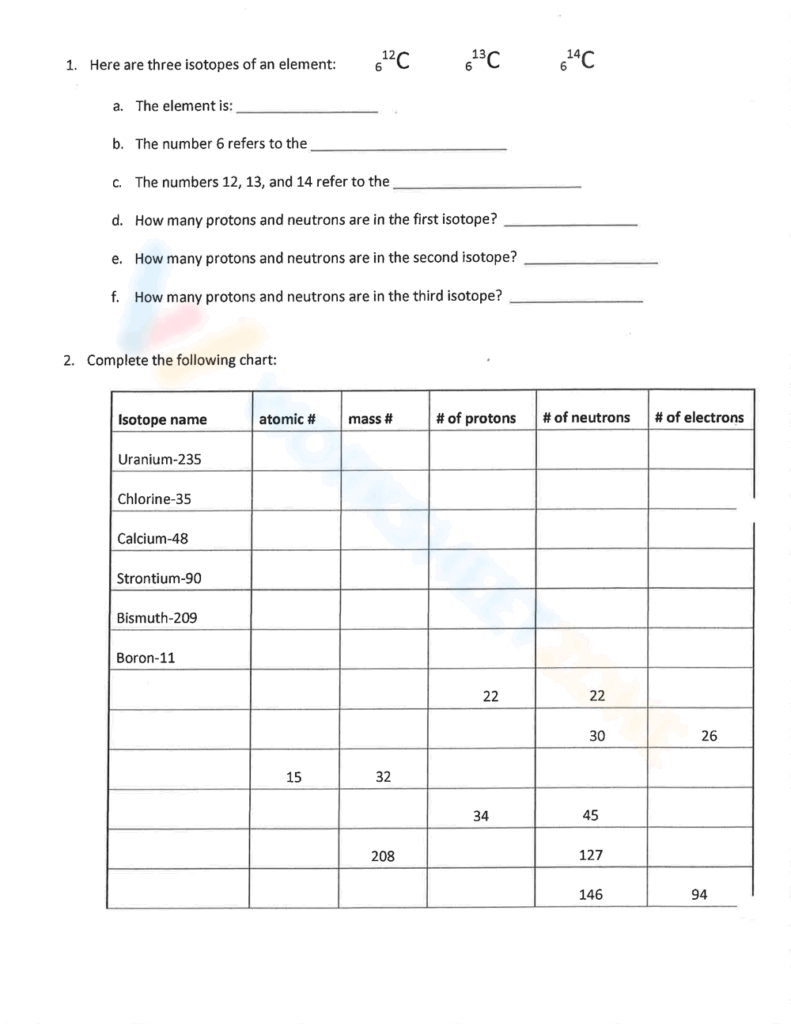
Atomic number and mass number are two fundamental concepts in chemistry that help us understand the structure of an atom. The atomic number of an element is the number of protons in its nucleus, which determines its identity. The mass number, on the other hand, is the total number of protons and neutrons in an atom’s nucleus.
When working with atomic number and mass number, it’s important to remember that the number of protons in an atom is always equal to its atomic number. The mass number, on the other hand, can vary for different isotopes of the same element.
Atomic Number Mass Number Worksheet
Using the Atomic Number Mass Number Worksheet
The Atomic Number Mass Number Worksheet is a valuable tool for practicing and reinforcing your understanding of these concepts. It typically consists of a series of questions and problems related to determining the atomic number and mass number of different elements.
By completing the worksheet, you can test your knowledge and skills in calculating atomic number and mass number, as well as identifying isotopes and understanding the significance of these numbers in chemistry. This hands-on practice can help solidify your understanding and improve your proficiency in working with atomic structure.
Conclusion
Overall, the Atomic Number Mass Number Worksheet is a useful resource for students and learners of chemistry to practice and enhance their understanding of these essential concepts. By actively engaging with the worksheet and solving the problems provided, you can strengthen your knowledge and skills in working with atomic number and mass number, ultimately improving your overall comprehension of atomic structure.